Topic 7. Equilibrium
7.1 Equilibrium
The equilibrium state is reached in a closed system when the rates of the forward and reverse reactions are equal.
-
the equilibrium is DYNAMIC since the forward and backward reactions are still occurring
-
concentrations of the reactants and products are CONSTANT, not necessarily equal
-
this is called the equilibrium mixture
-
illustrated in the figure to the right
-

Pearson textbook pg. 313
-
The equilibrium state is dynamic
-
The equilibrium state is achieved in a closed system - no exchange of matter with the surroundings
-
In the equilibrium state, the [reactants] and [products] remain constant
-
In the equilibrium state, there is no change in macroscopic properties
-
The equilibrium state can be reached from either direction (forward or backward)
The equilibrium constant Kc can be predicted from a reaction's stoichiometry

-
The product of the concentrations of the reactants becomes the denominator
-
The product of the concentrations of the products becomes the numerator
-
The coefficient becomes the "power" of its respective reactant/product
The magnitude of Kc gives information on the extent of reaction
A HIGH value of Kc → at equilibrium, there are proportionately MORE products than reactants
-
equilibrium mixture "lies to the right" = goes almost to completion
A LOW value of Kc → at equilibrium, there are proportionately LESS products than reactants
-
equilibrium mixture "lies to the left" = reaction has barely taken place

10⁻³ and 10³ are the benchmarks
-
if the Kc value is lower than 10⁻³, then the reaction is considered to lie to the left
-
if the Kc value is greater than 10³, then the reaction is considered to lie to the right
Pearson textbook pg. 317
The reaction quotient Q lets us predict the direction of the reaction
To calculate Q, use the same equation as Kc.
The main difference between Q and Kc is that Kc is basically Q at equilibrium.
-
Q: can be found at any time
-
Kc: can only be found at equilibrium
If Q = Kc → the reaction is at equilibrium → no net reaction occurs
If Q < Kc → the reaction proceeds to the right in favor of the products
If Q > Kc → the reaction proceeds to the left in favor of the reactants
-
this is because Q "wants to decrease" in order to go towards Kc
We can find the value of Kc for different expressions of a reaction
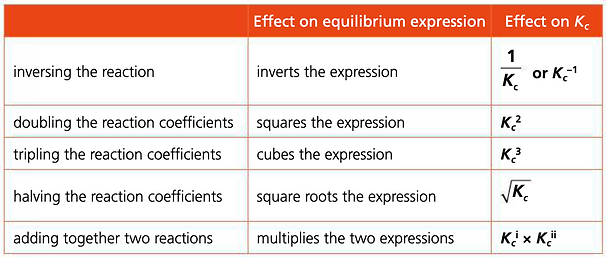
Pearson textbook pg. 320
What happens when the equilibrium is disrupted?
According to Le Chatelier's principle, a system at equilibrium when subjected to a change will respond in such a way as to minimize the effect of the change.
-
in other words, it will try to minimize the effect
1. changes in concentration
ex. N₂ (g) + 3H₂ (g) ⇌ 2NH₃ (g)
-
If the concentration of N₂ ↑, then the reaction will try to use it up → since N₂ is a reactant, the reaction goes to the right
-
If the concentration of N₂ ↓, then the reaction will try to make more → since N₂ is a reactant, the reaction goes to the left
2. changes in pressure (ONLY FOR GASES)
-
If pressure ↑, the equilibrium will shift to the side with a smaller # of molecules
-
If the pressure ↓, the equilibrium will shift to the side with a greater # of molecules
To determine the relative # of molecules: compare the sum of the mol ratios on either side
ex. N₂ (g) + 3H₂ (g) ⇌ 2NH₃ (g)
-
On the reactants side, there is a total of 4 moles
-
On the products side, there is a total of 2 moles
-
The reactants side has a greater # of molecules
-
If the # of moles is the same on each side → NO SHIFT in equilibrium
3. changes in volume (ONLY FOR GASES)
Remember that pressure and volume are inversely proportional. Mentally convert volume to pressure so you can utilize the knowledge from above.
4. changes in temperature
-
If the temp of the surroundings ↑, the reaction will try to decrease it by absorbing energy → favors the ENDOTHERMIC reaction
-
If the temp of the surroundings ↓, the reaction will try to increase it by releasing energy → favors the EXOTHERMIC reaction
For most equilibrium reactions, one direction (either forward or backward) is endothermic while the other is exothermic.
In the example equation below, the enthalpy change (∆H) value is given.
-
The ∆H is always given in terms of the forward reaction direction.
-
Since ∆H is negative, we know that the forward reaction is exothermic. Thus, the backward reaction is endothermic.

In this specific example,
-
If temp ↑, the eq shifts to the left (endothermic)
-
If temp ↓, the eq shifts to the right (exothermic)
5. addition of a catalyst → DOES NOT CHANGE THE EQ. POSITION, just changes the RATES
By definition, the catalyst will decrease the activation energy for both the forward and backward reactions
-
this is because both the forward and backward reactions pass through the same transition state
-
the catalyst makes both the forward and backward reactions "easier" by the same amount
-
thus, there is no shift in equilibrium position or Kc (no increase in equilibrium yield)
-
BUT the catalyst will speed up the attainment of the eq state and causes the products to form more quickly
summary table:

* only a change in temperature can change the value of Kc.
Pearson textbook pg. 325

The Haber process
The Haber process is an industrial synthesis of ammonia NH₃ from its elemental components N₂ and H₂.
-
enabled the production of explosives
-
revolutionized global food production
N₂ (g) + 3H₂ (g) ⇌ 2NH₃ (g) ∆H = -- 93 kJ/mol (exothermic)
-
all the reactants and products are gases
-
there is a change in the number of moles of gas molecules as the reaction proceeds ( 4 : 2 )
the optimum conditions for the industrial production of ammonia (high equilibrium yield):
-
high pressure: since the product side (NH₃) has a smaller # of molecules, the equilibrium will shift to the right, producing more ammonia
-
lower temperature: since the forward reaction is exothermic, the equilibrium will shift to the right, producing more ammonia
-
however, too low a temperature would cause the reaction to be uneconomically slow
-
moderate temperature of 450ºC is favored
-
-
catalysts: increases the rate of reaction
The Contact process
The Contact process is the production of sulfuric acid H₂SO₄.
-
three steps:
1. combustion of sulfur to form sulfur dioxide
2. oxidation of sulfur dioxide to sulfur trioxide: 2SO₂ (g) + O₂ (g) ⇌ 2SO₃ (g) ∆H = --196 kJ/mol (exothermic)
3. combination of sulfur trioxide with water to produce sulfuric acid
-
the overall rate depends on step 2, so the following conditions are optimized for the reaction in step 2.
the optimum conditions for the production of sulfuric acid (high equilibrium yield):
-
high pressure: since the product side (SO₃) has a smaller # of molecules, the equilibrium will shift to the right, producing more sulfur trioxide
-
lower temperature: since the forward reaction is exothermic, the equilibrium will shift to the right, producing more sulfur trioxide
-
however, too low a temperature would cause the reaction to be uneconomically slow
-
moderate temperature of 450ºC is favored
-
-
catalysts: increases the rate of reaction
-
ex. vanadium (V) oxide
-